Tuning the Body’s Defenses: LSU Researchers Build on Nobel Prize-Winning Science
October 09, 2025
When LSU immunology researcher Weishan Huang heard that the 2025 Nobel Prize in Physiology or Medicine was awarded for the discovery of regulatory T cells and the key gene FOXP3 that defines them, she wasn’t surprised.

Weishan Huang
She believes that broader recognition of this field and its pioneers is long overdue. The award signals growing momentum behind regulatory T-cell biology as a potential target for new therapeutics for cancer and inflammatory diseases. Huang is conducting research in this area at the LSU School of Veterinary Medicine, with promising findings.
“Regulatory T-cell discovery is highly deserving of the Nobel Prize,” Huang said. “The difficult part, I imagine, was deciding who to give the award to. Many people have made critical advances in this field. But I think the best decision was to give the award to the pioneers Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi, because they made the initial discoveries that have motivated the work of hundreds of research groups, including mine.”
Huang’s lab is working to identify signaling pathways that control not only the development but also the stability and activity of regulatory T cells.
The Tea on Regulatory T-Cells
Today, we know that all of us are born with regulatory T cells, called natural Tregs, which develop in a gland located in the upper part of the chest, known as the thymus. Tregs are a special type of T cell, a common group of cells that work for your body’s inner army: the immune system.
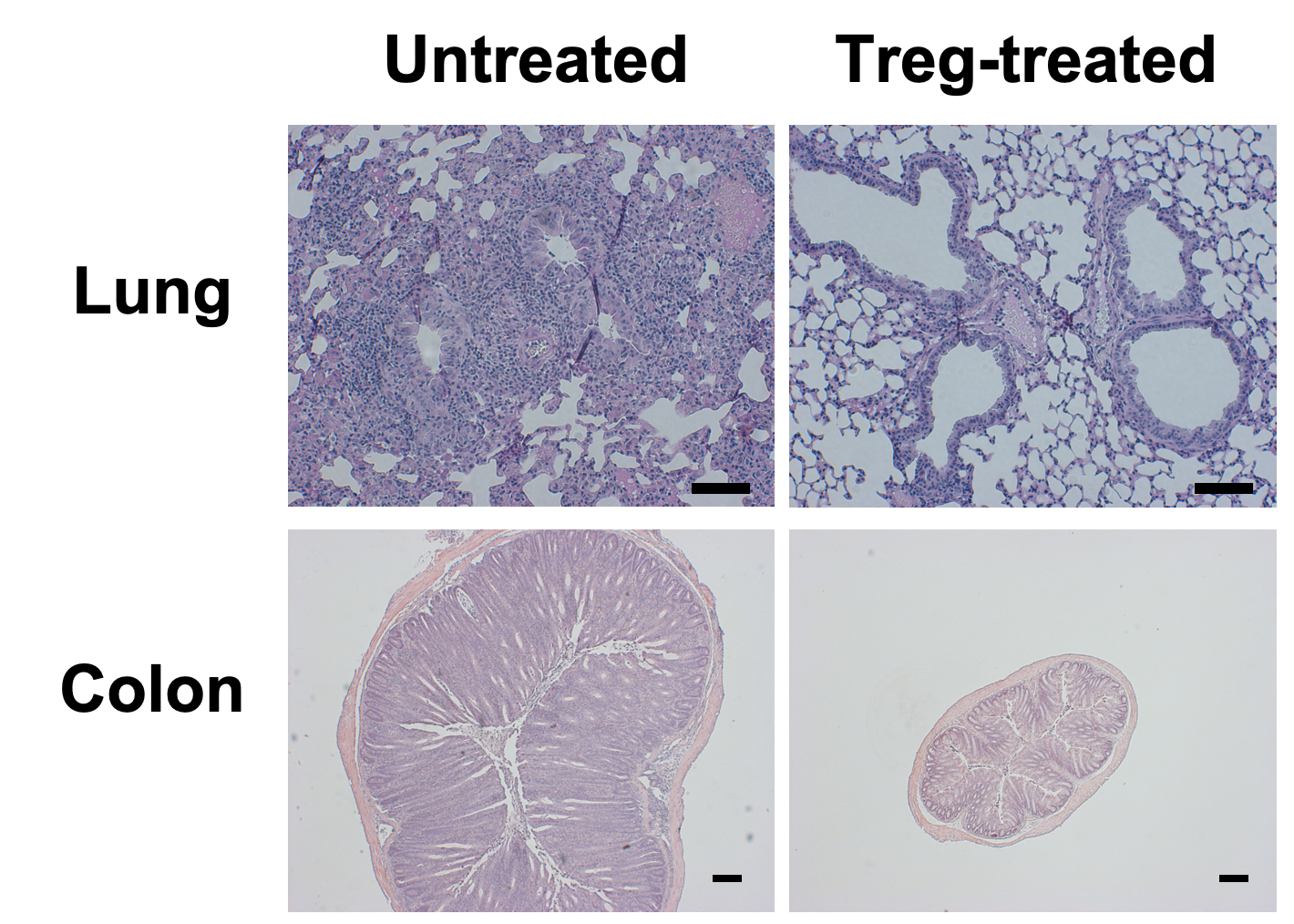
Views of lung and colon tissue under a microscope showing the therapeutic potential of Treg cells in inhibiting inflammation in the lung and gut. Treg-treated tissues show less inflammation.
As a whole, T cells help your body fend off pathogens and cancer. Tregs, however, are special T cells that suppress your immune system instead of helping it attack. Without these cells, we wouldn’t survive for long after birth. We’d experience massive inflammation and autoimmune diseases as our immune systems attacked our own cells.
On the other hand, too many regulatory T cells that are too suppressive can also create issues, especially when it comes to cancer.
“Cancer cells are very cunning,” Huang said. “A tumor, for example, can create an environment around itself that triggers blood vessel growth so that it can get more nutrients. It can also create a local environment that exhausts or suppresses immune cells, including T cells.”
A tumor can trick surrounding T cells into becoming its allies and acting as regulatory T cells (Tregs). As a result, the T cells don’t attack and kill the tumor cells. Instead, they in turn emit suppressive signals that tell other immune cells not to attack the tumor. Many researchers, including Huang, are working to find ways to remove these suppressive signals, allowing T cells to regain their normal defending activity.
To study Tregs and understand their impact on human health and disease, researchers first needed to identify and understand their origins. The 2025 Nobel Prize laureates did just that, identifying specific genes that control Treg development and showing that these genes are highly conserved in evolutionary history, being present in both mice and humans.
“Many researchers have followed up on these discoveries, using the FOXP3 gene to isolate and even create regulatory T cells for laboratory studies,” Huang says.
Nobel laureate Shimon Sakaguchi was the first to show that too few Tregs can lead to excessive inflammation and autoimmune diseases in animal models. Tregs help your body differentiate between what is “you” and what is from the outside or is “strange” and potentially harmful, such as viruses, bacteria, and even cancer cells. Regulatory T cells are immune suppressive, meaning they calm your broader immune system down so it doesn’t attack your own cells in its fight against invaders.
“You can actually collect Tregs from a healthy mouse, based on some of the markers the cells express, and then give them to a sick animal,” Huang says. “As a result, the sick animal has less inflammation. Experiments of this kind demonstrate that Tregs are truly regulatory and that they have therapeutic potential.”
From ‘All or Nothing’ to Turning a Dial
Despite garnering the 2025 Nobel Prize, regulatory T-cell biology has yet to deliver its first tested and approved drug or therapy. It’s not that we don’t have drugs that can target these cells, Huang explains. Similar to cancer drugs that kill both cancer cells and healthy cells, producing toxic side effects, existing means of suppressing Tregs also impact pathogen-fighting T cells that our immune system counts on.
“ It is a challenge, but we are on the hunt for specific signaling pathways that can precisely regulate the stability and activity of Treg cells. ”
In many ways, Tregs resemble and behave similarly to other T cells. Lamis El-Baz, a postdoctoral researcher in Huang’s lab, explains that you can’t differentiate Tregs and other T cells just by looking at them under a microscope. Their differences are invisible and consist of differing gene regulation and molecular pathways.
“It is extremely challenging to find the signals through which we can modulate regulatory T cells only, without a prevalent side effect,” Huang said. She and El-Baz are identifying signals that are more involved in controlling the activity of Tregs than other T cells. Their goal is to find molecular dials with which to tune Tregs activity, dialing it up or down. Being able to adjust Tregs' activity as needed would present opportunities to quell autoimmune diseases and inflammation on one hand, and boost immune system reactivity to cancer cells on the other.
“We want good control, or in scientific terms, regulatory immunity, to tell our immune system when to stay calm and be cool with more suppressive Tregs activity, and when to be less suppressive so that the immune system can unleash its full power against chronic infection or cancer,” Huang said.
So far, the lab has successfully identified two genes that help control the number of active regulatory T cells and their degree of suppressive activity. One of these genes, called SHMT2, is involved in helping our cells copy and repair their DNA, withstand chemical stressors, and divide and grow. This gene produces an enzyme that itself relies on vitamin B and folate to produce the essential building blocks of DNA. Studying this gene and the metabolic pathways associated with it, Huang and El-Baz observed something interesting: when they deleted this gene in Treg cells, the cells became less suppressive. The team hopes to use SHMT2 as a target to study how they can encourage T-cells to be more active in fighting cancer cells.
Ultimately, dialing T-cells to be calmer or more aggressive is a delicate balance.
“We need a way to dial down Tregs' suppressive activity without affecting all T cells, because if we just kill all of them in a patient, that person will be immunodeficient and could become fatally ill from a simple cold,” Huang said. “It is a challenge, but we are on the hunt for specific signaling pathways that can precisely regulate the stability and activity of Treg cells."
Future Possibilities
Huang and El-Baz are excited about the future of regulatory T-cell biology, especially in the wake of the 2025 Nobel Prize. They look forward to publishing a new mouse model to help other researchers make advances in understanding and modifying the activity of regulatory T cells in various disease conditions.
“One challenge is that Tregs don’t behave the same way in every disease or tissue. Their biology is very complex,” El-Baz said. “It will take a lot of effort and teamwork to map out how best to apply this potential therapeutic target in different conditions.”
Learn more about regulatory T-cell biology research at LSU.
Next Step
Discover stories showcasing LSU’s academic excellence, innovation, culture, and impact across Louisiana.


